
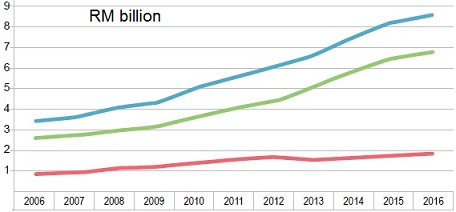
The Malaysian Market
From 2006 to 2016, the pharmaceutical market (prescription and over-the-counter (OTC)
drugs) grew at an average annual rate of 8.3% from RM3.4 billion to RM8.6 billion.
Suppliers
In order for suppliers to gain entry into the Malaysian market, the various methods include:
Direct entry, Representative office or appointing a local distributor. Direct entry entails
formation of a local company, otherwise required for the other two methods.
A representative office would require a locally appointed sales and marketing team with minimal
support staff. Most of the major pharmaceutical companies, with sufficient local turnover would
be able to opt for either one of the first two.
However, there are yet many suppliers with good, innovative products wishing to enter the local
market that can do neither, either due to cost constraints or lack of focus.
A third method allows such smaller companies or even larger companies who wish to maintain their
focus on their core products to still have local representation for their brands.
This is done throught agency/licensing (appoint and give rights to local company to represent
supplier with no local direct cost).
Regulatory Environment
In the 1980's, the Drug Control Authority was set up under the Control of Drugs and Cosmetics
Regulations 1984. The National Pharmaceutical
Regulatory Agency (current name) was given the task of ensuring the quality, efficacy and
safety of pharmaceuticals through the registration and licensing scheme.
The Medical Devices Authority was set up in the 2010's
under the Medical Device Act 2012, with full implemetation from July 2018.
Effectively with these two provisions, suppliers who supply goods in the market will have to comply
with the regulations of this country in order to ensure safety, efficacy and quality of the products
sold, for the benefit of the citizens.
Somedico' Role
With Somedico's long presence in the Malaysian market, we know the needs of the market and are familiar
with the regulatory landscape in Malaysia.
Using the knowledge and skills built over the years, Somedico is well placed to ensure a cost-effective
market entry into Malaysia, with expertise required to succeed in the various areas.
Our company infrastucture enables us to provide various services to our partners (see our partners), and would be able to represent new suppliers that have similar
needs and goals as Somedico's.
Sales
Our sales team are positioned to sell OTC healthcare, ethical pharmaceuticals, prescription only medications
and medical devices.
We are able to provide non-exclusive and exclusive sales teams for companies that wish to have representation
for their products. This is an ideal arrangement that makes market penetration in Malaysia affordable for the
supplier.
Marketing
Within Somedico's divisions, we provide marketing services for our partners, according to the channels of
distribution and confines of the regulatory environment.
Consumer marketing approach is provided by the OTC Health Care division. Ethical marketing is practised within
the Therapeutics Division targeting the medical and allied health care professionals.
Logistics
Somedico has a centralized warehousing, distribution and administration center that supports our logistics services.
Somedico's warehouse is GDP (Good Distribution Practise) compliant, as mandated by the National
Pharmaceutical Regulatory Agency and Medical Devices Authority. A separate
section for controlled medicines enhances the security and safety of our operations.
Within the Central region of Kuala Lumpur and its surrounding areas we have dedicated vans to transport customer purchases.
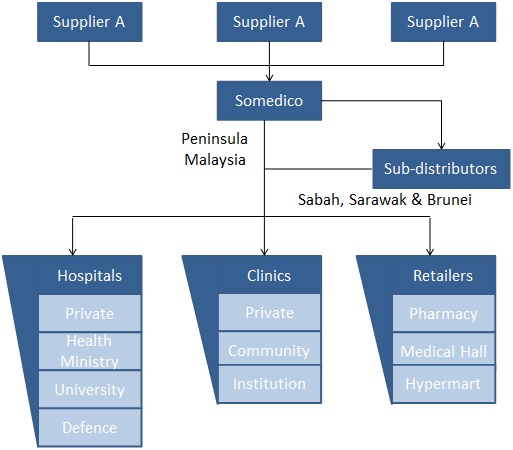
We have logistic partners for other areas within Peninsula Malaysia that provide the transportation support.
In addition to this, we have sub-distributors in Sabah, Sarawak and Brunei, to augment our logistics operations.
All matters relating to invoicing and credit control is handled at our main office.
Regulatory Affairs
Our regulatory department is fully equipped to register products as required by the Drug Control Authority(DCA). Somedico
has experience in the registration of:
- New Chemical Entities (NCE's)
- Controlled Medicines defined under the Poisons act 1952
- Over the counter medicines (OTC)
- Toiletries/Cosmetics
- Health supplements
- Helbal/Traditional Medicines
- Medical Devices
- Medical Foods
Somedico provides service to register products either under its own name or on behalf of the product licence holder.
|